Stainless steel is widely used in industrial applications due to its excellent corrosion resistance; however, in chlorine-containing environments, it is susceptible to pitting and localized dissolution, which can compromise equipment safety. This paper explores the corrosion mechanisms and protection technologies of 316L metal valves, reviews recent research progress in corrosion prevention, and examines advanced methods such as surface modification and synergistic use of corrosion inhibitors. These approaches aim to enhance the safety and durability of industrial equipment and provide theoretical support for the development of new corrosion-resistant valve materials.
Metal valves are critical components in industrial process control, particularly in highly corrosive environments such as petrochemical plants, nuclear power facilities, and marine engineering. Their performance is directly linked to the safe operation of the entire system. 316L stainless steel exhibits excellent resistance to intergranular corrosion, thanks to the addition of 2.5% molybdenum and a controlled carbon content below 0.03%. It is widely used in applications such as LNG transportation, nuclear reactor cooling, and other demanding environments. In 2023, the global market for 316L stainless steel reached USD 4.86 billion. However, real-world engineering cases have shown that 316L valves remain vulnerable to corrosion under extreme conditions such as high chloride concentrations, elevated temperatures, and high humidity. For example, stress corrosion cracking has been observed in the bellows of pressure safety valves at petrochemical plants, and severe crevice corrosion occurs at flange connections in the South China Sea environment, resulting in average annual economic losses exceeding 780 million US dollars. It is evident that advancing corrosion protection technologies is crucial for enhancing valve reliability and extending service life.
Zhong and colleagues investigated the chloride-induced corrosion behavior of 304 stainless steel at 25 °C and 80 °C. As the temperature increases, the resistance of the passivation film gradually decreases, and the pitting potential also declines. Sun investigated the effects of chloride ions (Cl⁻), oxygen (O₂), and carbon monoxide (CO) on the corrosion resistance of the passivation film on 316L stainless steel. The study found that Cl⁻ accelerates the anodic dissolution of the metal, disrupts the structure and density of the passivation film, and significantly reduces its corrosion resistance. Lin Li studied the corrosion behavior of 316L stainless steel under various influencing factors in a high-temperature, chlorine-containing environment, and concluded that as the ambient temperature increases, the passivation film becomes more unstable and more susceptible to chloride-induced corrosion. Kexi Liao's research indicated that once the critical pitting temperature is exceeded, pitting begins to form on the surface of the sample. Moreover, the higher the chloride ion concentration, the lower the critical pitting temperature of 316L stainless steel and the greater the number of pits observed on the surface. The above research findings demonstrate that stainless steel exhibits poor pitting corrosion resistance in high-temperature, high-chloride environments. Enhancing its resistance to pitting and reducing the associated corrosion risks are therefore of great significance to engineering practice.
Current research on the corrosion mechanisms of 316L metal valves primarily focuses on localized corrosion in specific environments. Through failure case analysis, Panda et al. reported that in chloride-containing media, 316L valve bellows are prone to stress corrosion cracking—particularly under conditions of 60 °C and 500 ppm chloride concentration—where the crack propagation rate can reach as high as 3.2 × 10⁻⁹ m/s.
The microstructure of stress corrosion cracking in 316L valve bellows is shown in Figure 1. Under chloride conditions, the stress corrosion cracking process can be divided into three main stages: crack initiation, intergranular propagation, and eventual through-wall leakage. Chloride-induced intergranular corrosion initiates crack formation, as shown in Figure 1. In (a), pitting occurs along grain boundaries on the outer surface, with cracks forming in stress concentration areas. The presence of martensite promotes grain boundary dissolution. In (b), branch cracks form, leading to intergranular fracture. In (c), secondary cracks appear along with the accumulation of corrosion products. Finally, in (d), full wall-thickness cracks develop, resulting in macroscopic leakage channels.
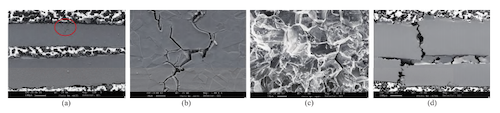
(a) Crack initiation area on the outer surface (circled) (b) Enlarged view of branch cracks
(c) Intergranular propagation mode of secondary cracks (d) Full wall-thickness cracks
Figure 1 Characteristics of chloride-induced intergranular crack propagation at different stages
Dianyu Liu’s team found that the pitting density of 316L stainless steel in deep ocean waters is significantly higher than in shallow waters, largely due to the formation of microbial films and localized anoxic microenvironments. Therefore, surface modification technology has become a key focus of corrosion protection research. The Co-based laser cladding coating developed by Zhang Yanchao demonstrates excellent performance in liquid Pb-Bi corrosion environments, reducing the annual corrosion rate by 90% compared to the substrate; however, the risk of interfacial cracking still requires attention. Although significant progress has been made in developing experimental methodologies, most studies remain limited to laboratory simulations, which differ substantially from real-world operating conditions. According to statistics, field-measured data on valve corrosion over the past five years account for only 12.7% of total research, leading to a gap between theoretical models and practical engineering applications. Regarding protection technology, the superhydrophobic micro-nano nickel coating developed by Jiang Bin achieves a contact angle of up to 162°, but its wear resistance in environments containing solid particles remains inadequate. Most existing coating systems are designed to address individual corrosion factors, and in-depth research on the synergistic mechanisms of CO-HS-Cl⁻ multiphase corrosion, commonly encountered in oil and gas fields, remains lacking.
Liquid nitriding technology significantly enhances surface hardness, wear resistance, and corrosion resistance by forming a nitride layer on the metal surface. The process has evolved from the highly toxic cyanide salt system to an environmentally friendly cyanate system, establishing a comprehensive technical framework. Currently, the three main liquid nitriding methods dominating the global market are Germany’s Degussa Tenifer process, France’s HEF Sursulf sulfur-nitrogen-carbon composite nitriding process, and China’s independently developed QPQ technology. The current process typically uses a medium-temperature treatment range of 550–600°C. While it improves surface properties, it often leads to the formation of precipitate phases, reduces corrosion resistance, and makes it difficult to simultaneously meet the requirements for high dimensional accuracy and mechanical performance. The improved QPQ technology developed by Colin Company in the United States has significantly enhanced surface roughness control, corrosion resistance, and wear resistance, offering new solutions for high-end manufacturing. Studies have shown that the corrosion rate of 316L stainless steel in fracturing return fluid containing 80 g/L chloride ions is 0.127 mm/a, highlighting the aggravating effect of a high Cl⁻ environment on pitting corrosion. Electrochemical impedance spectroscopy reveals that micro-nano nickel plating increases the charge transfer resistance by two orders of magnitude. In-situ Kelvin probe measurements also confirmed that the potential difference at the valve stem thread reaches 120 mV, marking the initiation point of crevice corrosion. In terms of surface treatment, laser cladding is an advanced technology that employs high-energy laser beams to functionally modify material surfaces. It offers advantages such as high energy density, precise process control, and a minimal heat-affected zone. This technology has been widely applied in aircraft engine blade repair, automotive gear strengthening, precision mold hardening, and the preparation of corrosion-resistant coatings for energy equipment. Among these, the Co-based alloy cladding layer offers excellent corrosion and wear resistance, along with multifunctional integration potential. By optimizing the alloy composition and introducing functional phases, it can achieve a combination of corrosion resistance, thermal conductivity, and electrical conductivity. In research conducted by Zhang Yanchao’s team at Jiangsu University, the Co-based coating exhibited a weight loss rate after 3,000 hours of corrosion in a liquid lead-bismuth environment that was only 18% of that of the substrate, with a microhardness reaching as high as HV 3580, demonstrating promising application potential despite equipment costs being approximately 40% higher than those of traditional processes. Additionally, Luo Jing prepared an Fe-Al coating using arc ion plating for high-temperature molten salt environments. The Fe, Al, and trace Cr elements in the outer diffusion layer react with oxygen ions in the molten salt to form a dense oxide layer, which effectively inhibits ion penetration. This reduces the corrosion rate of 316L valves in 650°C carbonate environments to 0.032 mg/(cm²·h), improving corrosion resistance by approximately 5.7 times. A summary of various protection methods is provided in Table 2.
Table 2 Metal corrosion protection methods
|
Protection Method |
Treatment Process |
Corrosion Environment |
Corrosion Rate |
Hardness (HV) |
Protection Efficiency |
Advantages & Disadvantages |
|
Salt Bath Nitriding |
Active nitrogen atoms from cyanate decomposition diffuse into the metal surface, forming a nitrided layer. |
Chloride-containing seawater |
Some alloying elements promote passivation, reducing corrosion rate. |
450–500 |
60% |
Good fluidity of molten salt at High temperatures ensures uniform nitriding; use of cyanide/cyanate liquids may pose environmental and safety concerns. |
|
Laser Cladding |
Co-based alloy laser cladding with Stellite-F alloy layer. |
Liquid Pb-Bi (300°C) |
Annual corrosion rate reduced by 90% |
580 |
90% |
Resistant to high-temperature liquid metal corrosion, and strong interface bonding; however, equipment costs are 40% higher than traditional methods. |
316L stainless steel valves are vulnerable to various forms of corrosion in complex environments involving chloride ions, high temperatures, and dynamic fluids. Data from coastal chemical plants indicate that a medium containing 500 ppm chloride ions can cause the pitting corrosion rate of 316L valves to reach 0.12 mm/year at 60°C, reducing their service life to just 18 months. Intergranular corrosion cracks up to 50 μm wide easily develop in high-temperature acidic environments. In LNG high-pressure transmission systems, liquid flow rates of 8 m/s cause valve cores to wear by more than 200 μm annually, leading to groove corrosion. Existing protection methods have clear limitations: passivation treatment is ineffective in strong acid environments; cathodic protection struggles to cover complex structures; plasma nitriding improves hardness but reduces corrosion resistance at welds; and laser cladding, while effective, is prone to micro-galvanic corrosion at the interface. These bottlenecks seriously compromise the long-term reliability of valves in extreme operating conditions, making the development of breakthrough protection technologies urgently necessary.
With increasingly complex operating conditions and stricter environmental regulations, corrosion protection technology for 316L metal valves is advancing toward greater diversity and intelligence. At the material level, high-entropy alloy coatings exhibit excellent corrosion resistance—for example, the pitting potential of AlCrFeCoNi coatings in 3.5% NaCl solution increases to 0.98 V, which is three times higher than that of traditional 316L stainless steel. Nanocomposite coatings, such as graphene-enhanced polyaniline, reduce corrosion rates to 0.002 mm/a, achieving protection efficiencies of up to 99.5%. Intelligent monitoring technologies are rapidly maturing; according to a NACE report, machine learning–based corrosion prediction models have an error rate as low as 7.3%, and real-time corrosion monitoring is now possible with IoT sensors. Green corrosion inhibitors have also made significant advances—for instance, bio-based imidazoline derivatives developed by the Chinese Academy of Sciences demonstrate 92% inhibition efficiency in high-temperature, high-pressure CO₂ environments, along with biodegradation rates exceeding 80%. Furthermore, the integration of interdisciplinary technologies—such as combining phase field simulation with electrochemical impedance spectroscopy—provides robust theoretical support for corrosion prediction and practical engineering applications.
Table 3 Comparison of different corrosion inhibitors
|
Corrosion Inhibitor Type |
Mechanism of Action |
Applicable Environment |
Corrosion Inhibition Efficiency |
Synergistic Effect |
Environmental Impact |
|
Chromates |
Anodic passivation film formation |
Neutral aqueous solution |
85% – 90% |
Single anodic protection; poor synergy with cathodic protection |
Toxic, biodegradation rate <10% |
|
Synthetic Imidazolidine Derivative |
Adsorption film + inhibition of cathodic reaction |
High temperature, high-pressure CO₂ environment (300°C) |
90% |
Synergizes with surface coatings to improve film density |
Low toxicity, degradation rate 60% |
|
Bio-based Imidazoline Derivatives |
Molecular targeted adsorption + enzymatic degradation |
Chloride-containing acidic medium (60°C) |
92% |
Competes with microbial films to reduce pitting initiation |
Non-toxic, degradation rate >80% |
|
Graphene-Polyaniline Composite |
Nanobarrier + enhanced electrochemical impedance |
Multiphase corrosion (CO₂, H₂S, Cl⁻) |
99.5% |
Synergistic inhibition of anodic dissolution and cathodic hydrogen reduction |
Environmentally friendly, and no secondary pollution |
Although 316L stainless steel valves exhibit excellent corrosion resistance, they still face significant challenges under complex operating conditions. In environments where Cl⁻ concentrations exceed 500 ppm, pitting, crevice corrosion, and stress corrosion cracking frequently occur, with corrosion risks especially pronounced on offshore platforms and in high-temperature molten salt systems. Surface protection technologies, such as FeAl coatings and laser-cladded Co-based coatings, can effectively reduce corrosion rates; however, limitations still remain. For example, salt bath nitriding treatments still result in uniform corrosion in acidic media, while super-hydrophobic coatings lack sufficient durability in flowing environments. Electrochemical nitriding layers tend to peel off in multiphase flows. Therefore, future efforts should focus on developing comprehensive corrosion prediction models that integrate materials, environments, and operating conditions, as well as advancing new protection technologies—such as intelligent adaptive coatings—to enhance the service reliability of 316L valves under extreme conditions.